Company Overview
AbbVie Statistics: AbbVie Inc. is a global biopharmaceutical company recognized for its diverse and innovative portfolio spanning immunology, oncology, neuroscience, aesthetics, and eye care. The company combines scientific expertise, a dedicated workforce, and a strong commitment to innovation to develop advanced therapies that target some of the world’s most challenging health conditions. Established as an independent, publicly traded entity on January 1, 2013, AbbVie emerged from the separation of Abbott Laboratories, which distributed 100% of AbbVie’s common stock to its shareholders.
The company operates through a unified global business segment. The company focuses on research, development, manufacturing, and commercialization of cutting-edge medicines and therapies. With a presence in over 70 countries, AbbVie’s research network spans 17 nations, including the US, France, Italy, Spain, Japan, and India, where more than 14,000+ scientists and professionals work toward discovering new treatments for a healthier future.
Key Facts
- AbbVie was formed in January 2013 as a spin-off from Abbott Laboratories, allowing it to focus entirely on biopharmaceutical innovation.
- The company’s name combines “Abbott” and the Latin word “vie,” meaning “life,” symbolizing its dedication to improving lives through science.
- AbbVie is headquartered in North Chicago, Illinois, and operates in more than 170 countries worldwide.
- It employs over 50,000 people globally, emphasizing science-driven healthcare solutions.
- AbbVie focuses on key therapeutic areas such as immunology, oncology, neuroscience, eye care, and aesthetics.
- The company’s mission is to discover and deliver innovative medicines and solutions that address serious health challenges.
- Since its formation, AbbVie has invested over $50 billion in research and development.
- Its most well-known product, Humira (adalimumab), was the world’s best-selling drug for several consecutive years.
- AbbVie acquired Allergan in 2019 for around $63 billion, expanding its portfolio in aesthetics and eye care.
- The company maintains a strong commitment to ESG principles, community engagement, and sustainable healthcare innovation.
History of AbbVie
- January 2013: AbbVie began its journey as an independent biopharmaceutical company after separating from Abbott Laboratories, officially debuting on the New York Stock Exchange at $34.40 per share.
- April 2013: The company produced and distributed its first AbbVie-branded product, manufacturing around 23,000 cartons within the first 24 hours.
- May 2013: AbbVie received its first S. FDA breakthrough therapy designation for a hepatitis C treatment, underscoring its strong focus on research and innovation.
- October 2013: AbbVie was ranked 4th on Science Magazine’s list of Top Science Employers, a notable achievement within its inaugural year.
- November 2013: Introduced “The AbbVie Way,” a unified cultural philosophy designed to enhance collaboration, performance, and innovation.
- June 2014: Launched its first Week of Possibilities, engaging over 7,000 employees across 60 countries in community service activities.
- September 2014: Formed a pioneering collaboration with Calico to advance research in ageing-related diseases.
- December 2014: Achieved S. FDA approval for its first independently developed treatment for chronic HCV, marking a key R&D milestone.
- October 2015: AbbVie’s therapy IMBRUVICA received the Prix Galien Award for Best Pharmaceutical Agent, one of the industry’s most prestigious honours.
- May 2016: Expanded neuroscience research by opening the Cambridge Research Center in Massachusetts, with a focus on Alzheimer’s disease.
- September 2016: Topped the biotech sector in the Dow Jones Sustainability World Index, leading in 12 of 22 evaluation criteria.
- June 2017: Named the Most Reputable Global Pharma Company by Reputation Institute, based on 16,500+ ratings across eight countries.
- July 2017: Gained FDA, EU, and Japan approvals for MAVIRET/MAVYRET, reinforcing its leadership in hepatitis treatment.
Further
- August 2018: Donated $100 million to Ronald McDonald House Charities, representing the largest single contribution in both organizations’ history.
- November 2018: Granted S. FDA accelerated approval for a novel therapy addressing acute myeloid leukemia (AML).
- May 2019: Supported education by donating $40 million to rebuild North Chicago’s Neal Math and Science Academy.
- August 2019: Received FDA and EU approvals for a plaque psoriasis therapy serving more than 125 million patients globally.
- May 2020: Finalized the acquisition of Allergan, strengthening its portfolio in aesthetics and ophthalmology.
- June 2020: Donated $5 million to support racial justice causes and pledged an additional $50 million over five years to community organizations.
- October 2020: Unveiled Allergan Aesthetics’ global brand identity, promoting inclusivity and empowerment across diverse audiences.
- October 2021: Ranked #4 on FORTUNE’s World’s Best Workplaces, climbing 11 positions from 2020, supported by over 48,000 employees.
- March 2022: Inaugurated a 480,000 sq. ft. R&D facility in the Bay Area, enhancing oncology research and collaboration.
- June 2022: Reinstated Week of Possibilities, mobilizing 15,000 employees across 50 countries for global volunteer work.
- November 2022: Donated $40million to establish a new STEM-focused public school in North Chicago, reinforcing community engagement.
- January 2023: Celebrated its 10th anniversary, marking over $50 billion invested in research and development to advance global healthcare innovation.
(Source: Company Website)
Statistics of AbbVie Financial Analysis
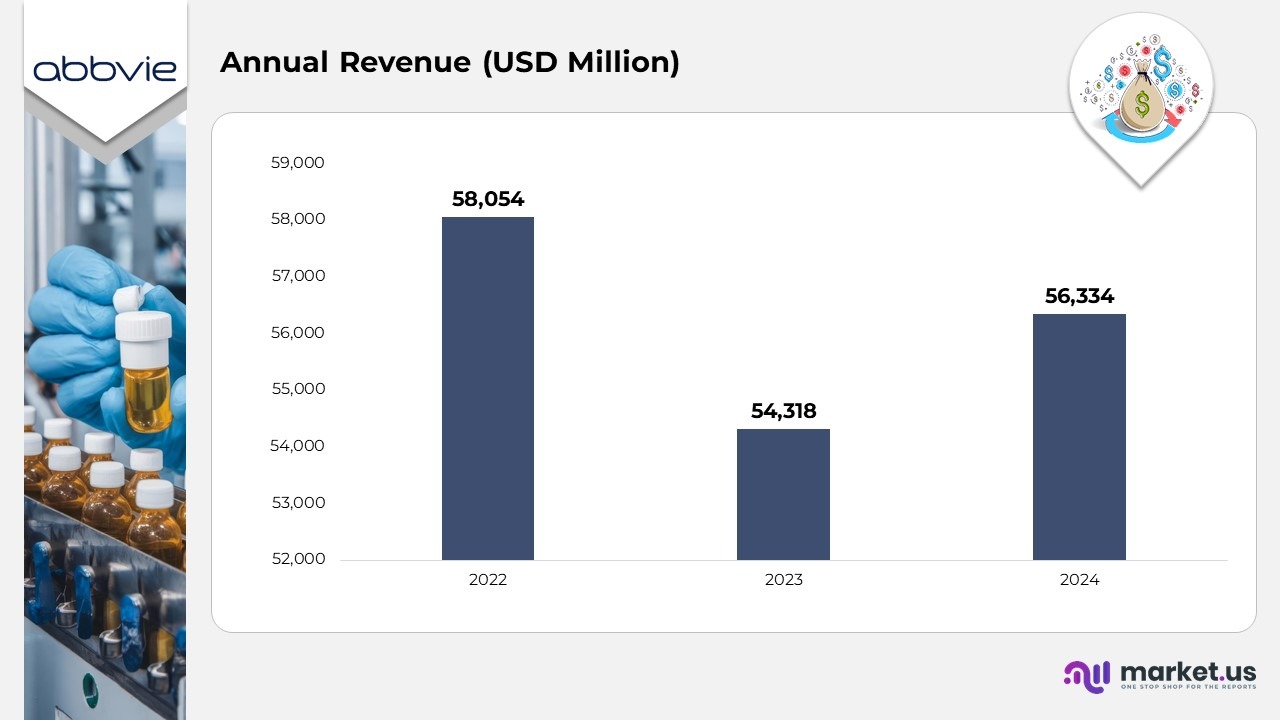
- In 2022, AbbVie achieved revenues of $58,054 million, reflecting strong momentum across its global therapeutic divisions and a solid execution of its growth strategy.
- In 2023, the company posted revenues of $54,318 million, indicating a 4% year-on-year decrease, primarily influenced by product lifecycle adjustments and intensified competitive dynamics.
- In 2024, AbbVie’s total revenue improved to $56,334 million, marking a 7% increase from the previous year, showcasing a steady recovery and renewed business growth.
- The company’s rebound was anchored by its strategic emphasis on 5 core segments: neuroscience, oncology, immunology, aesthetics, and eye care, which collectively enhanced overall portfolio stability and performance.
- During 2024, AbbVie’s leading immunology therapies, Rinvoq and Skyrizi, delivered a combined revenue exceeding $17.6 billion, underscoring the company’s sustained dominance in the global immunology market.
Geographical Revenue By Statistics of AbbVie
- In 2024, AbbVie’s revenue in the United States totaled $43,029 million, up from $41,883 million in 2023, though slightly below $45,713 million in 2022. The U.S. remained AbbVie’s largest and most profitable market, led by robust performance in immunology and neuroscience
- In Germany, revenue reached $1,465 million in 2024, rising from $1,266 million in 2023 and $1,340 million in 2022, reflecting a stable and resilient performance across AbbVie’s European operations.
- In Japan, sales increased to $1,122 million in 2024, compared to $1,008 million in 2023 and $956 million in 2022, supported by growing demand for speciality and chronic care medicines.
- In Canada, AbbVie generated $1,088 million in 2024, a slight improvement over $1,076 million in 2023, though marginally below $1,159 million in 2022, maintaining steady performance across North America.
- In China, total revenue stood at $917 million in 2024, compared with $950 million in 2023 and $912 million in 2022, indicating consistent operations amid a dynamic healthcare policy environment.
- In France, AbbVie recorded $776 million in 2024, nearly unchanged from $780 million in 2023 and $787 million in 2022, showing sustained regional stability in product demand.
Further
- In Spain, revenue improved to $528 million in 2024 from $501 million in 2023, with a small gain over $506 million in 2022, reflecting continued uptake of key therapeutic products.
- In the United Kingdom, sales rose sharply to $522 million in 2024, from $417 million in 2023 and $462 million in 2022, marking a strong recovery and improved market conditions.
- In Italy, AbbVie reported $511 million in 2024, up from $484 million in 2023 and $444 million in 2022, indicating gradual growth in Southern European markets.
- In Brazil, the company achieved $464 million in 2024, compared to $439 million in 2023 and $430 million in 2022, showing steady expansion across Latin America.
- In Australia, revenue reached $463 million in 2024, slightly below $472 million in 2023 and $508 million in 2022, reflecting a consistent and mature market presence.
- Across all other countries, AbbVie recorded $5,449 million in 2024, compared to $5,042 million in 2023 and $4,837 million in 2022, showcasing continued global diversification and solid international growth.
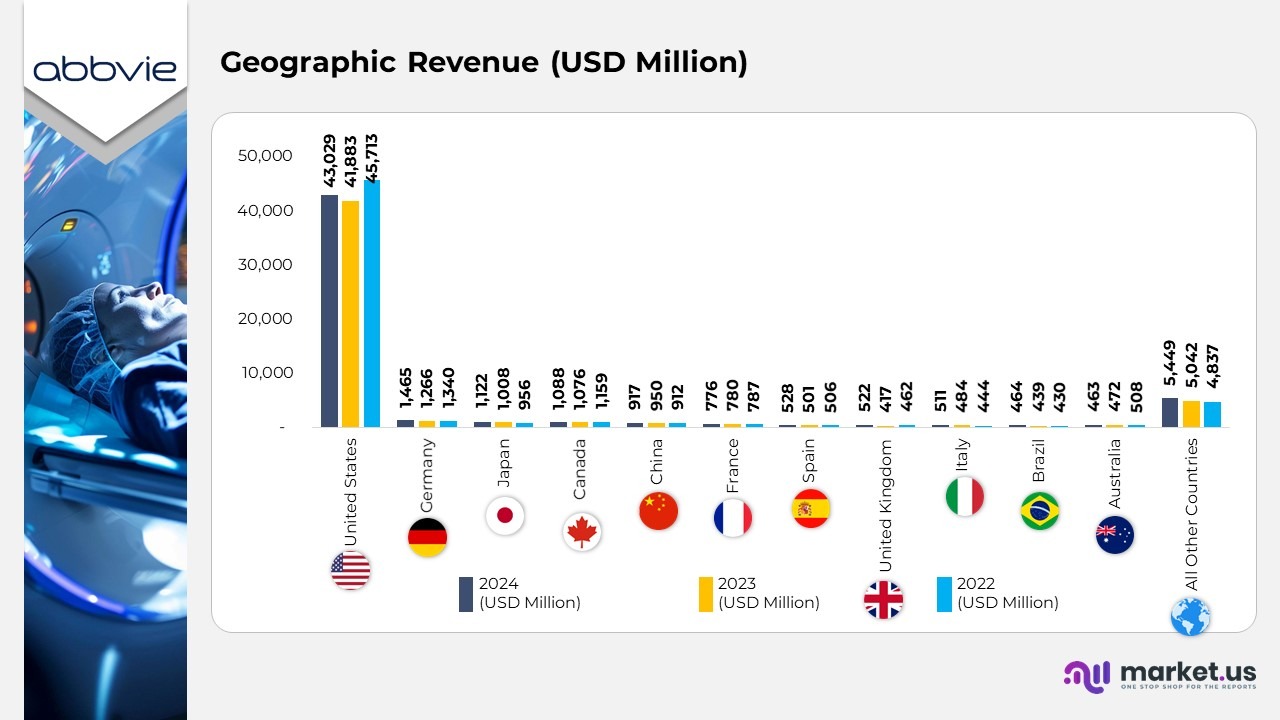
(Source: AbbVie SEC Filings)
Research and Development Expenditure
- Research and Development (R&D) expenses represented a higher percentage of AbbVie’s net revenues in 2024 compared to 2023, indicating an increased focus on innovation and portfolio expansion.
- The 2024 R&D ratio was adversely influenced by a significant $4.5 billion intangible asset impairment charge associated with emraclidine, compared to a smaller $630 million impairment recorded in 2023.
- Increased investment was directed toward advancing projects across all stages of the company’s research and development pipeline, reflecting AbbVie’s commitment to strengthening its future therapeutic portfolio.
- The rise in R&D spending also included acquisition-related and integration expenses tied to the ImmunoGen and Cerevel Therapeutics transactions, covering cash-settled post-closing costs for employee incentive programs.
- Further details regarding these expenses and impairment charges are available in Note 5 of the consolidated financial statements.
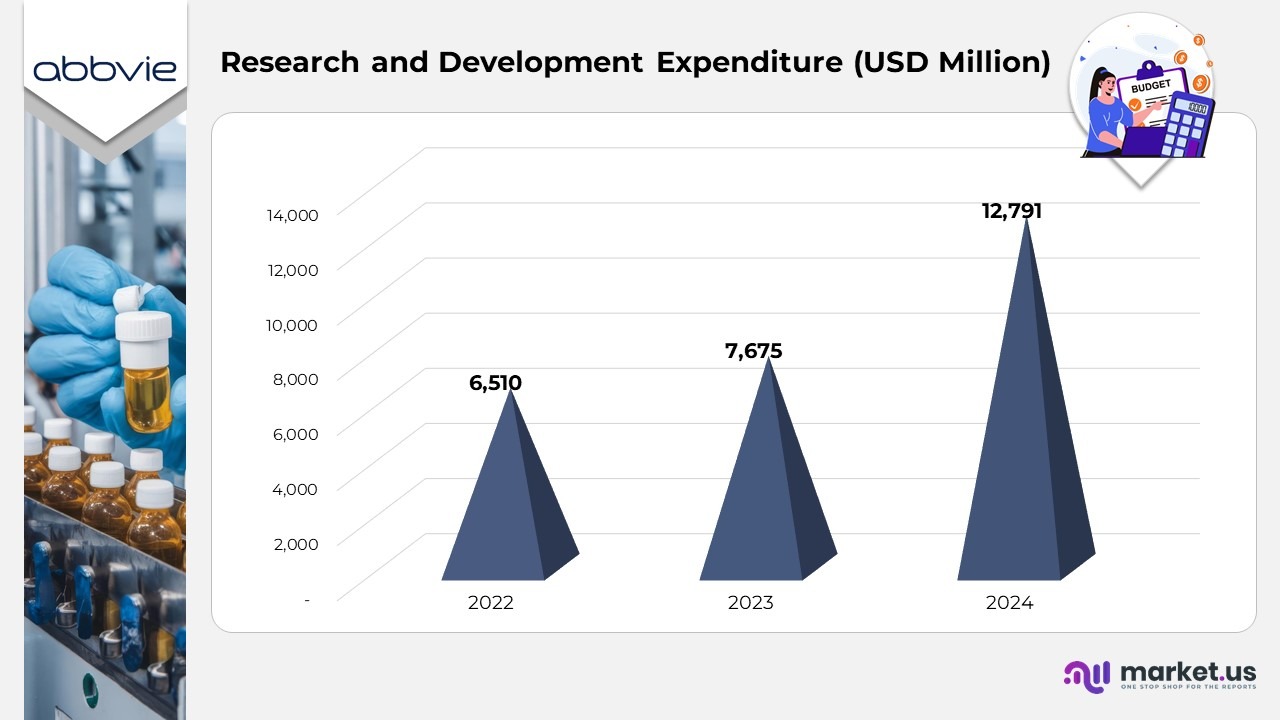
(Source: AbbVie SEC Filings)
AbbVie Product-Wise Revenue Performance Statistics
- In 2024, AbbVie’s Botox Therapeutic recorded $2,718 million in U.S. revenue, increasing from $2,476 million in 2023 and $2,255 million in 2022, driven by consistent neurological treatment demand. International sales reached $565 million, up from $515 million in 2023 and $464 million in 2022, bringing the total to $3,283 million globally.
- Vraylar generated $3,260 million in the U.S. during 2024, up from $2,755 million in 2023 and $2,037 million in 2022, supported by broader patient access and higher prescriptions. International sales were $7 million in 2024, compared to $4 million in 2023 and $1 million in 2022, taking total revenue to $3,267 million.
- Duodopa achieved $96 million in the U.S. in 2024, nearly unchanged from $97 million in 2023, while international revenue stood at $351 million, slightly below $371 million in 2023 and $363 million in 2022, totalling $447 million overall.
- Ubrelvy reported $981 million in U.S. revenue in 2024, an increase from $803 million in 2023 and $680 million in 2022, indicating expanding migraine therapy adoption. International performance improved to $25 million in 2024 from $12 million in 2023, totalling $1,006 million worldwide.
- Qulipta reached $628 million in U.S. sales during 2024, up from $405 million in 2023 and $158 million in 2022, driven by strong uptake in migraine management. International revenue grew to $30 million from $3 million, bringing total global sales to $658 million.
- Other Neuroscience products contributed $224 million in the U.S. during 2024, slightly lower than $254 million in 2023, while international sales rose to $114 million from $22 million, taking the combined total to $338 million.
- Ozurdex reported $138 million in U.S. sales in 2024, compared to $143 million in 2023 and $139 million in 2022. International revenue climbed to $356 million from $329 million, with total revenue reaching $494 million.
Moreover
- Lumigan/Ganfort achieved $187 million in the U.S. during 2024, up from $173 million in 2023, while international markets delivered $242 million, compared to $259 million in 2023 and $272 million in 2022, for a total of $429 million.
- Alphagan/Combigan posted $95 million in U.S. sales in 2024, down from $121 million in 2023, whereas international performance improved slightly to $153 million from $151 million, resulting in a total of $248 million.
- Restasis generated $172 million in U.S. revenue in 2024, declining from $382 million in 2023 and $621 million in 2022 due to growing generic competition. International sales amounted to $52 million, contributing to total revenue of $224 million.
- Other Eye Care products delivered $472 million in U.S. revenue in 2024, increasing from $433 million in 2023 and $399 million in 2022. International sales reached $375 million, up slightly from $370 million, resulting in $847 million in total revenue.
- Mavyret posted $595 million in U.S. sales in 2024, lower than $659 million in 2023 and $755 million in 2022, while international markets earned $716 million, compared to $771 million the previous year, leading to total revenue of $1,311 million.
- Creon revenue in the U.S. reached $1,383 million in 2024, up from $1,268 million in 2023 and $1,278 million in 2022, supported by continued demand in pancreatic enzyme therapies.
- Linzess/Constella recorded $916 million in U.S. sales in 2024, down from $1,073 million in 2023, while international performance rose slightly to $38 million from $35 million, maintaining steady overall growth.
(Source: AbbVie SEC Filings)
AbbVie Aesthetic and Therapeutic Product U.S. Patent Portfolio Statistics
| Product | Patent Number (U.S.) |
|---|---|
| ALLODERM RTU | 8,735,054 |
| COOLSCULPTING | 8,285,390; 8,523,927; 8,702,774; 9,132,031; 9,375,345; D777,338; 9,655,770; 9,861,421; 10,292,859; 10,524,956; 10,568,759; 10,675,176; 10,675,178; 10,912,599; 11,076,879; 11,179,269; 11,219,549; 11,224,536; 11,291,606; 11,583,438; 11,819,257; 11,986,421 |
| COOLTONE | D1,028,258; 12,048,851 |
| DiamondGlow | 8,128,638; 8,236,008; 8,945,104; 9,050,133; 9,655,432; 9,775,645; 9,918,727; 10,485,983; 10,492,807; 10,898,227; 11,013,534 |
| FOURTE Expander Fill System | 10,433,928 |
| JUVEDERM Ultra Plus XC | 8,357,795; 8,450,475; 8,822,676; 9,089,518; 9,089,519; 9,358,322; 10,391,202; 10,485,896; 10,596,321; 11,020,512; 11,173,232; D865,948; D865,949; D865,950; D866,753; D867,582 |
| JUVEDERM Ultra XC | 8,357,795; 8,450,475; 8,822,676; 9,089,518; 9,089,519; 9,358,322; 10,391,202; 10,485,896; 10,596,321; 11,020,512; 11,173,232; D865,948; D865,949; D865,950; D866,753; D867,582 |
| JUVEDERM VOLBELLA XC | 7,741,476; 8,357,795; 8,450,475; 8,822,676; 9,089,517; 9,089,518; 9,089,519; 9,358,322; 10,391,202; 10,485,896; 10,596,321; 11,020,512; D865,948; D865,949; D865,950; D866,753; D867,582 |
| JUVEDERM VOLLURE XC | 7,741,476; 8,357,795; 8,450,475; 8,822,676; 9,089,517; 9,089,518; 9,089,519; 9,358,322; 10,391,202; 10,485,896; 10,596,321; 11,020,512; D865,948; D865,949; D865,950; D866,753; D867,582 |
| JUVEDERM VOLUMA | 7,741,476; 8,357,795; 8,822,676; 9,089,517; 9,089,518; 9,089,519; 10,485,896; 10,596,321; D865,948; D865,949; D865,950; D866,753; D867,582 |
| JUVEDERM VOLUX XC | 7,741,476; 8,357,795; 8,450,475; 8,822,676; 9,089,517; 9,089,518; 9,089,519; 9,358,322; 10,391,202; 10,485,896; 10,596,321; 11,020,512; 12,324,868; D865,948; D865,949; D865,950; D866,753; D867,582 |
| KELLER FUNNEL (Silicone Breast Implant Sleeve) | 8,211,173; 8,550,090; 8,555,893; 9,402,713; 10,213,294; 10,463,472 |
| KYBELLA (deoxycholic acid) injection, for subcutaneous use | 7,622,130; 7,754,230; 8,101,593; 8,242,294; 8,367,649; 8,461,140; 8,546,367; 8,653,058; 8,883,770; 9,522,155; 9,636,349; 9,949,986; 10,500,214 |
| OCUMEND | 10,022,335 |
| OPTIVE Fusion UD | 8,569,367 |
| REFRESH OPTIVE Advanced | 9,314,528; 8,569,367; 8,957,048; 9,907,826; 10,105,386; 10,888,598 |
| REFRESH OPTIVE 0.5% & 0.9% (Lubricant Eye Drops) | 8,569,367 |
(Source: Company Website)
Fun Facts
- Although founded in 2013, AbbVie’s heritage dates back to Abbott Laboratories, which began in 1888, giving it over 135 years of scientific legacy.
- AbbVie’s culture emphasizes collaboration, innovation, and agility, often summarized in mottos like “Make Possibilities Real.”
- The company’s blockbuster drug Humira has been prescribed to millions of patients globally for autoimmune diseases such as rheumatoid arthritis and Crohn’s disease.
- AbbVie is one of the few pharmaceutical firms that successfully transitioned from dependence on a single blockbuster drug (Humira) to a diversified portfolio of next-generation therapies.
- Its acquisition of Allergan added iconic brands such as Botox and Juvederm to its portfolio, strengthening its aesthetics division.
- AbbVie ranks consistently among the top global pharma innovators due to its continuous focus on cutting-edge biologics and targeted therapies.
- Employees across its global network participate in AbbVie’s annual “Week of Possibilities,” a volunteer initiative supporting local communities.
- The company integrates advanced technologies like AI and data analytics to accelerate clinical development and improve patient outcomes.
- AbbVie celebrates “Science for All” initiatives to promote STEM education and diversity in scientific careers.
- Despite being a young company by legal age, AbbVie stands among the top pharmaceutical giants globally in revenue, R&D spend, and innovation output.
Recent Developments
- In October 2025, AbbVie completed the acquisition of Gilgamesh Pharmaceuticals, aimed at accelerating the development of next-generation compounds for serious mental health disorders. This move underscores AbbVie’s continued focus on advancing innovative, science-based treatment options in neuroscience.
- In October 2025, the S. Food and Drug Administration (FDA) approved a supplemental new drug application (sNDA) for Rinvoq (upadacitinib), expanding its indication to include treatment of adults with moderately to severely active ulcerative colitis (UC) and Crohn’s disease (CD), further strengthening AbbVie’s immunology portfolio.
- In September 2025, AbbVie announced a $70 million expansion project to enhance its biologics manufacturing and R&D capabilities in the United States, reinforcing its long-term commitment to scientific innovation and domestic production.
- In September 2025, the company submitted a Biologics License Application (BLA) to the FDA seeking approval for Pivekimab sunirine (PVEK), an investigational therapy for Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN), a rare and aggressive hematologic malignancy.
- In September 2025, AbbVie launched the “Naturally You with Injectable Hyaluronic Acid Fillers” campaign, focused on providing transparent and educational information about HA injectable fillers to counter misinformation and highlight their safe, natural-looking results.
Moreover
- In August 2025, AbbVie acquired Capstan Therapeutics, gaining access to a possible in vivo targeted lipid nanoparticle (tLNP) anti-CD19 CAR-T therapy and a proprietary tLNP RNA delivery platform, enhancing its leadership in autoimmune disease and genetic medicine research.
- In August 2025, the company introduced the SkinMedica HA5 Hydra Collagen Hydrating Foaming Cleanser, featuring the brand’s proprietary HA5 Hydra Collagen Complex, a blend of five types of hyaluronic acid and vegan collagen designed to hydrate and refresh the skin deeply.
- In August 2025, AbbVie announced a $195 million investment in a new S. manufacturing plant to expand domestic active pharmaceutical ingredient (API) production. This initiative forms part of AbbVie’s larger $10 billion U.S. capital investment plan to boost innovation and manufacturing capacity.
- In July 2025, AbbVie received FDA approval for the fixed-duration, all-oral combination regimen of Venclexta (venetoclax) and acalabrutinib for previously untreated chronic lymphocytic leukemia (CLL) patients, offering another time-limited therapeutic option within its oncology portfolio.
- In June 2025, the FDA approved a label expansion for Mavyret (glecaprevir/pibrentasvir), authorizing its use in adults and children aged three years and older with acute or chronic hepatitis C virus (HCV). This makes Mavyret the first and only DAA therapy approved for acute HCV, achieving a 96% cure rate in just eight weeks.
- In May 2025, AbbVie announced a partnership with the Chicago Cubs to launch the “Striking Out Cancer” initiative, supporting cancer awareness and research. As part of the collaboration, AbbVie pledged to donate $233 for every strikeout by a Cubs pitcher during the 2025 regular season, symbolizing the 233 Americans diagnosed with cancer every hour, as reported by the American Cancer Society.
(Source: AbbVie Press Releases)