Global Enzyme Replacement Therapy Market By Product Type (Imiglucerase, Agalsidase Beta, Taliglucerase, Velaglucerase Alfa, and Others), By Route of Administration (Parenteral and Oral), By Therapeutic Condition (Gaucher Disease, Pompe Disease, SCID, Fabry Disease, and Others), By End-user (Infusion Centers, Hospitals, and Others), Region and Companies – Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2025-2034
- Published date: June 2025
- Report ID: 151464
- Number of Pages: 350
- Format:
-
keyboard_arrow_up
Quick Navigation
- Report Overview
- Key Takeaways
- Product Type Analysis
- Route of Administration Analysis
- Therapeutic Condition Analysis
- End-User Analysis
- Key Market Segments
- Drivers
- Restraints
- Opportunities
- Impact of Macroeconomic / Geopolitical Factors
- Latest Trends
- Regional Analysis
- Key Players Analysis
- Recent Developments
- Report Scope
Report Overview
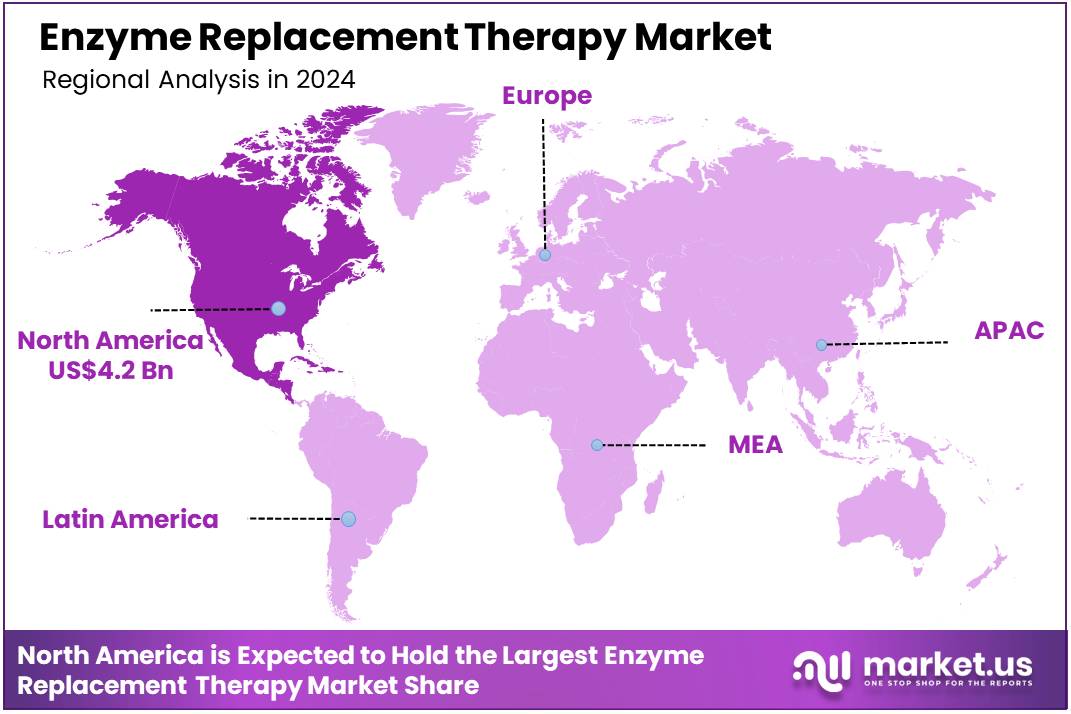
Global Enzyme Replacement Therapy Market size is expected to be worth around US$ 25.1 Billion by 2034 from US$ 10.5 Billion in 2024, growing at a CAGR of 9.1% during the forecast period 2025 to 2034. In 2024, North America led the market, achieving over 39.8% share with a revenue of US$ 4.2 Billion.
Growing recognition of rare genetic disorders and the need for targeted treatments are driving the expansion of the enzyme replacement therapy (ERT) market. ERT has proven to be a game-changer for patients with inherited enzyme deficiencies, offering life-changing solutions for conditions like Gaucher disease, Fabry disease, and Pompe disease. These therapies work by providing patients with the deficient enzymes, thereby addressing the root cause of these disorders and preventing severe complications such as organ damage, pain, and neurological decline.

As a result, demand for ERT continues to rise, with increasing investments in research and development to explore new indications and improve treatment efficacy. Recent trends in the market show a growing focus on personalized therapies that cater to the unique needs of individual patients. Advances in gene therapy and next-generation enzyme products further boost market prospects.
In January 2024, JR-441, an experimental enzyme replacement therapy for Sanfilippo syndrome type A, received Orphan Drug Designation from the FDA. Developed by JCR Pharmaceuticals, JR-441 targets the deficient heparan N-sulfatase enzyme, which is essential for breaking down heparan sulfate. A Phase 1/2 trial in Germany is evaluating its safety, tolerability, and effects on neurological symptoms, highlighting the ongoing innovation in enzyme replacement therapies and their expanding applications for rare diseases. These developments present significant opportunities for growth in the ERT market.
Key Takeaways
- In 2024, the market for enzyme replacement therapy generated a revenue of US$ 10.5 Billion, with a CAGR of 9.1%, and is expected to reach US$ 25.1 Billion by the year 2034.
- The product type segment is divided into imiglucerase, agalsidase beta, taliglucerase, velaglucerase alfa, and others, with imiglucerase taking the lead in 2023 with a market share of 39.8%.
- Considering route of administration, the market is divided into parenteral and oral. Among these, gaucher disease held a significant share of 81.4%.
- Furthermore, concerning the therapeutic condition segment, the market is segregated into gaucher disease, pompe disease, SCID, fabry disease, and others. The parenteral sector stands out as the dominant player, holding the largest revenue share of 52.3% in the enzyme replacement therapy market.
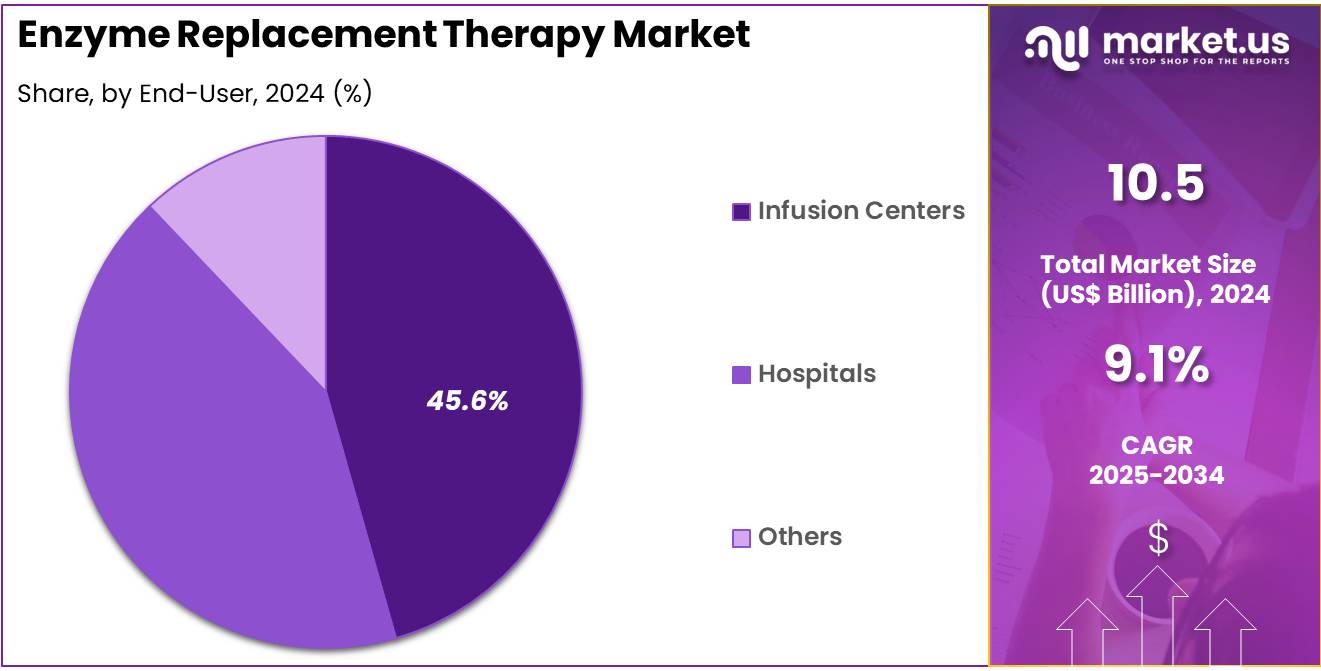
- The end-user segment is segregated into infusion centers, hospitals, and others, with the infusion centers segment leading the market, holding a revenue share of 45.6%.
- North America led the market by securing a market share of 39.8% in 2023.
Product Type Analysis
Imiglucerase remains the dominant product in the enzyme replacement therapy market, accounting for 39.8% of the market share. Imiglucerase is widely used for treating Gaucher disease, one of the most common lysosomal storage disorders. Its established efficacy and long track record of use have cemented its position as a primary treatment option. The growth of this segment is expected to be driven by its continued adoption in clinical settings, especially as more patients with Gaucher disease seek long-term management solutions.
Additionally, the development of biosimilars and advancements in the formulation of imiglucerase are anticipated to enhance patient access and affordability, further driving market demand. As awareness of Gaucher disease and its treatment options increases, imiglucerase is projected to maintain its dominance in the enzyme replacement therapy market.
Route of Administration Analysis
Parenteral administration is projected to remain the leading route of administration in the enzyme replacement therapy market, holding 81.4% of the share. Parenteral administration, which includes intravenous and subcutaneous injections, is essential for delivering enzyme replacement therapies due to the need for precise and consistent drug delivery. The effectiveness of parenteral therapies in managing severe enzyme deficiencies makes them the preferred choice for most ERT treatments.
As clinical evidence supporting the efficacy and safety of parenteral enzyme replacement therapies continues to grow, their adoption in both hospital and outpatient settings is expected to increase. The significant prevalence of conditions like Gaucher disease, which requires ongoing parenteral therapy, will continue to drive the demand for these treatments. Additionally, the growing number of infusion centers offering specialized parenteral ERT services is likely to contribute to the continued dominance of parenteral administration in the market.
Therapeutic Condition Analysis
Gaucher disease is expected to remain the largest therapeutic condition in the enzyme replacement therapy market, comprising 52.3% of the market share. Gaucher disease, a rare genetic disorder caused by a deficiency in the enzyme glucocerebrosidase, has a significant patient population requiring lifelong enzyme replacement therapy. The segment’s growth is expected to be driven by the increasing diagnosis of Gaucher disease and the growing availability of effective treatments such as imiglucerase and other ERT products.
Advances in genetic testing and the increased awareness of Gaucher disease are anticipated to lead to earlier diagnosis and treatment, further expanding the market for enzyme replacement therapies. The rising number of specialized treatment centers and healthcare providers focused on Gaucher disease will likely continue to drive the adoption of enzyme replacement therapies for this condition, ensuring its dominant position in the market.
End-User Analysis
Infusion centers are projected to be the dominant end-user in the enzyme replacement therapy market, accounting for 45.6% of the market share. Infusion centers, which specialize in administering intravenous therapies, are expected to see significant growth as the demand for enzyme replacement therapies, particularly for conditions like Gaucher disease, increases. These centers provide patients with specialized care, allowing for the safe administration of parenteral therapies in a controlled environment.
The rise in outpatient care, as well as the increasing number of patients opting for home infusion services, is anticipated to boost the growth of infusion centers. Moreover, as the healthcare system continues to focus on improving patient access to specialized treatments, infusion centers will remain key players in the distribution and administration of enzyme replacement therapies, ensuring their continued growth in the market.
Key Market Segments
By Product Type
- Imiglucerase
- Agalsidase Beta
- Taliglucerase
- Velaglucerase Alfa
- Others
By Route of Administration
- Parenteral
- Oral
By Therapeutic Condition
- Gaucher Disease
- Pompe Disease
- SCID
- Fabry Disease
- Others
By End-user
- Infusion Centers
- Hospitals
- Others
Drivers
Increasing Diagnosis and Awareness of Lysosomal Storage Disorders is Driving the Market
The rising global awareness and improved diagnostic capabilities for various lysosomal storage disorders (LSDs), which are often treated with enzyme replacement therapy, are primary drivers for the market. Enhanced newborn screening programs and genetic testing are leading to earlier and more accurate diagnoses, allowing for timely initiation of ERT, which can significantly improve patient outcomes and quality of life.
Lysosomal storage disorders, as a group, have a combined incidence estimated to be between 1 in 5,000 to 1 in 8,000 births worldwide, as reported in a July 2022 study in the National Library of Medicine. Conditions like Pompe disease, which is a specific type of LSD treated with ERT, affects approximately 1 in 40,000 individuals in the United States, as per the National Institute of Health in April 2022. This growing understanding and ability to identify patients with these rare genetic conditions are expanding the addressable patient population for ERT.
Restraints
High Cost of Treatment and Limited Access in Developing Regions are Restraining the Market
The enzyme replacement therapy market faces significant restraint due to the exceptionally high cost of these specialized treatments and persistent challenges with accessibility, particularly in developing regions. ERT medications are often among the most expensive drugs, placing a substantial financial burden on healthcare systems, insurance providers, and individual patients. For example, treatments for rare diseases, which include many conditions managed by ERT, can cost hundreds of thousands of US dollars annually per patient.
While specific ERT cost data from government sources are often not aggregated, the Indian Organization for Rare Diseases (IORD) reported in May 2022 that the Indian government increased its financial grant for rare disease treatment from US$24,000 (INR 20 lakh) to US$60,000 (INR 50 lakh), highlighting the high financial need for such therapies. This high price point and the complex reimbursement processes in many countries can limit widespread adoption and create disparities in patient access, despite the life-saving nature of these therapies.
Opportunities
Advancements in Gene Therapy and Next-Generation ERTs Create Growth Opportunities
Ongoing advancements in gene therapy research and the development of next-generation enzyme replacement therapies present significant growth opportunities in the market. Gene therapy aims to provide a potentially curative solution by correcting the underlying genetic defect, thereby enabling the body to produce the missing enzyme itself, potentially reducing or eliminating the need for lifelong infusions. The US Food and Drug Administration (FDA) in November 2024 approved Kebilidi (eladocagene exuparvovec-tneq), a gene therapy for AADC deficiency, a rare genetic disorder characterized by an enzyme deficiency, marking a significant step forward in gene therapy for enzyme-related conditions.
Furthermore, research into modified enzymes with improved pharmacokinetics, targeted delivery, and reduced immunogenicity promises to enhance the efficacy and convenience of existing ERTs. These innovations are poised to expand treatment options, improve patient compliance, and potentially transform the landscape for individuals with enzyme deficiencies.
Impact of Macroeconomic / Geopolitical Factors
Macroeconomic factors significantly influence the enzyme replacement therapy market, primarily through their direct impact on national healthcare budgets, pharmaceutical pricing, and R&D investment in rare diseases. In economically prosperous periods, governments and private health insurers are typically more willing to allocate substantial funds for expensive, life-saving treatments like ERT, leading to broader access and faster adoption of new therapies.
Conversely, economic downturns or periods of high inflation can force austerity measures in healthcare spending, potentially leading to stricter reimbursement criteria or delays in access to these high-cost drugs. The World Health Organization (WHO) reported in December 2024 that global health spending saw a real-terms decline in 2022, which can present challenges for the funding of rare disease treatments. Geopolitical factors, such as trade policies, international intellectual property rights, and the stability of global supply chains for highly specialized biologic manufacturing, also play a critical role.
Disruptions caused by geopolitical tensions, as seen in 2024 with various trade and logistics issues, can increase manufacturing costs, lead to shortages of essential raw materials or components, and delay the distribution of these complex therapies. However, the high unmet medical need and the devastating progression of untreated rare enzyme deficiencies often compel continued prioritization and investment in this market, offering a degree of resilience despite external economic and political pressures.
Current US tariff policies can directly impact the enzyme replacement therapy market by altering the cost of imported raw materials, highly specialized cell culture media, and advanced bioprocessing equipment crucial for manufacturing biologic drugs. Given that many ERTs are complex biologics, their production relies on intricate global supply chains, often involving specialized components sourced internationally.
The Budget Lab at Yale University projected in May 2025 that a 25% ad valorem tariff on pharmaceuticals could increase medication costs by an average of around US$600 per year per household in the US, indicating the broad potential impact of such policies on drug pricing. In 2024, US imports of medicinal and pharmaceutical products totaled US$234 billion, with major contributions from countries like Ireland, Switzerland, and Germany, as reported by Voronoi in February 2025.
Any tariffs imposed on these key import categories would directly increase manufacturing expenses for companies producing ERTs. This could translate to higher prices for patients and healthcare systems, potentially limiting access to these vital therapies.
Conversely, such tariff policies can incentivize pharmaceutical companies to invest more heavily in establishing or expanding domestic manufacturing capabilities for ERTs within the US. This strategic shift towards localized production could lead to a more secure and resilient supply chain for these critical medicines, reducing dependence on potentially volatile international sources and enhancing national health security in the long term, despite the immediate challenges of increased costs and the need for significant capital investment.
Latest Trends
Increased Focus on Newborn Screening Programs for Early Detection is a Recent Trend
A prominent recent trend in the enzyme replacement therapy market is the intensified focus on expanding and implementing comprehensive newborn screening programs for various metabolic disorders, many of which can be managed by ERT if detected early. Early diagnosis through these screenings allows for the initiation of treatment before irreversible organ damage occurs, leading to significantly better clinical outcomes for affected infants.
The National Institutes of Health (NIH) continues to support research into new screening technologies and the inclusion of additional conditions in recommended uniform screening panels. For instance, in 2023-2024, several states in the US added new conditions to their newborn screening programs, reflecting a nationwide effort to broaden early detection for rare genetic disorders, including those treatable by ERT. This proactive approach to identifying and treating patients early drives demand for ERT products and enhances their therapeutic impact.
Regional Analysis
North America is leading the Enzyme Replacement Therapy Market
North America dominated the market with the highest revenue share of 39.8% owing to the ongoing need to manage rare genetic disorders and the introduction of new therapeutic options. Lysosomal storage disorders (LSDs), a group of inherited metabolic diseases often treated with ERT, affect a significant number of individuals; for example, according to the National Institute of Health, approximately 1 in 5,000 newborns is affected by an LSD.
While specific year-over-year prevalence data for 2022-2024 from direct government sources is generally not yet published in aggregated form, the consistent incidence of these conditions ensures a steady patient base requiring lifelong treatment.
Furthermore, regulatory approvals play a crucial role in market expansion; in February 2023, the US Food and Drug Administration (FDA) approved Lamzede (velmanase alfa-tycv) for the treatment of non-central nervous system manifestations of alpha-mannosidosis, and in May 2023, Elfabrio (pegunigalsidase alfa-iwxj) received FDA approval for confirmed Fabry disease. These approvals expand the available treatment landscape for specific lysosomal storage disorders, offering new options for patients and contributing to the overall market’s growth.
The Asia Pacific region is expected to experience the highest CAGR during the forecast period
Asia Pacific is expected to grow with the fastest CAGR owing to increasing awareness of rare genetic disorders, improving diagnostic capabilities, and a rising focus on enhancing healthcare infrastructure. While precise prevalence rates for all lysosomal storage disorders from direct government sources across every Asia Pacific country for 2022-2024 are complex to aggregate, countries like China are actively cataloging rare diseases and approving new treatments.
For instance, the “Incidence and prevalence of 121 rare diseases in China: Current status and challenges: 2022 revision,” a study published in a journal associated with the National Health Commission of China, highlights ongoing efforts to identify and manage rare diseases.
The Asia Pacific region is expected to benefit from increased healthcare spending, as indicated by the “Health at a Glance: Asia/Pacific 2024” report from the OECD and WHO, which reflects a broader investment in health services. This improved access to diagnostics and treatment, combined with governmental and international health initiatives aimed at rare diseases, is projected to fuel the expansion of access to enzyme replacement therapies throughout Asia Pacific.
Key Regions and Countries
North America
- US
- Canada
Europe
- Germany
- France
- The UK
- Spain
- Italy
- Russia
- Netherland
- Rest of Europe
Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- New Zealand
- Singapore
- Thailand
- Vietnam
- Rest of APAC
Latin America
- Brazil
- Mexico
- Rest of Latin America
Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Rest of MEA
Key Players Analysis
Key players in the enzyme replacement therapy (ERT) market focus on expanding their treatment offerings by developing novel therapies for a wider range of rare genetic disorders. They prioritize advancing their research and development efforts to improve the efficacy and safety profiles of existing treatments. Strategic partnerships with biopharmaceutical companies, research institutions, and healthcare providers help these companies strengthen their market position. Furthermore, key players actively seek regulatory approvals for new indications, allowing them to target new patient populations. Expanding patient access through global distribution channels and patient support programs also plays a vital role in their growth strategies.
Sanofi Genzyme is a leading player in the enzyme replacement therapy market. As a part of Sanofi, Genzyme focuses on developing treatments for rare genetic diseases, including Lysosomal Storage Disorders (LSDs). The company’s flagship product, Cerdelga, is an ERT treatment for Gaucher disease, one of the most common LSDs. With a strong emphasis on research and patient support, Sanofi Genzyme works closely with healthcare professionals to ensure that patients receive timely and effective treatments. The company continues to expand its portfolio through ongoing research, collaborations, and clinical trials to address unmet medical needs in rare disease areas.
Top Key Players
- Recordati Rare Diseases
- Protalix Biotherapeutics
- Horizon Pharma Public Limited Company
- Denali Therapeutics, Inc
- Biomarin Pharmaceutical Inc
- Amicus Therapeutics
- Actelion (Janssen)
- AbbVie
Recent Developments
- In January 2025, Denali Therapeutics, Inc. revealed that the US FDA granted Breakthrough Therapy Designation to its investigational enzyme replacement therapy, DNL310 (tividenofusp alfa), for Hunter syndrome (MPS II). This therapy delivers IDS enzymes to both the brain and body to alleviate the symptoms of the disease.
- In September 2023, Amicus Therapeutics received FDA approval for its two-component therapy, Pombiliti (cipaglucosidase alfa) and Opfolda (miglustat), for adults with Pompe disease who are not improving on existing enzyme replacement therapies. This innovative combination therapy enhances enzyme uptake and stabilizes activity in the blood, addressing critical needs within the Pompe community.
Report Scope
Report Features Description Market Value (2024) US$ 10.5 Billion Forecast Revenue (2034) US$ 25.1 Billion CAGR (2025-2034) 9.1% Base Year for Estimation 2024 Historic Period 2020-2023 Forecast Period 2025-2034 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Product Type (Imiglucerase, Agalsidase Beta, Taliglucerase, Velaglucerase Alfa, and Others), By Route of Administration (Parenteral and Oral), By Therapeutic Condition (Gaucher Disease, Pompe Disease, SCID, Fabry Disease, and Others), By End-user (Infusion Centers, Hospitals, and Others) Regional Analysis North America – US, Canada; Europe – Germany, France, The UK, Spain, Italy, Russia, Netherlands, Rest of Europe; Asia Pacific – China, Japan, South Korea, India, Australia, New Zealand, Singapore, Thailand, Vietnam, Rest of APAC; Latin America – Brazil, Mexico, Rest of Latin America; Middle East & Africa – South Africa, Saudi Arabia, UAE, Rest of MEA Competitive Landscape Recordati Rare Diseases, Protalix Biotherapeutics, Horizon Pharma Public Limited Company, Denali Therapeutics, Inc, Biomarin Pharmaceutical Inc, Amicus Therapeutics, Actelion (Janssen), AbbVie Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF)  Enzyme Replacement Therapy MarketPublished date: June 2025add_shopping_cartBuy Now get_appDownload Sample
Enzyme Replacement Therapy MarketPublished date: June 2025add_shopping_cartBuy Now get_appDownload Sample -
-
- Recordati Rare Diseases
- Protalix Biotherapeutics
- Horizon Pharma Public Limited Company
- Denali Therapeutics, Inc
- Biomarin Pharmaceutical Inc
- Amicus Therapeutics
- Actelion (Janssen)
- AbbVie










