Global Cimzia (certolizumab) Drug Market Analysis By Indication (Psoriatic Arthritis (PsA), Rheumatoid Arthritis (RA), Other Indications), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), By Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2024-2033
- Published date: March 2024
- Report ID: 47706
- Number of Pages: 297
- Format:
-
keyboard_arrow_up
Quick Navigation
Report Overview
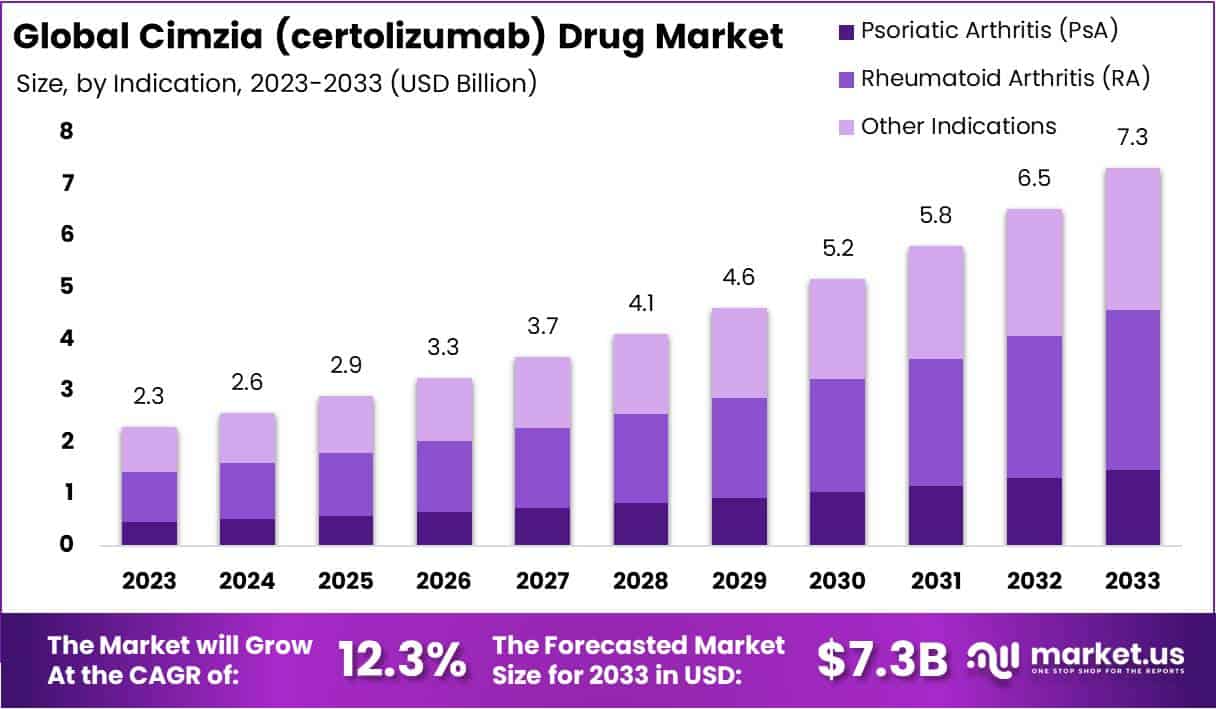
The Global Cimzia (certolizumab) Drug Market size is expected to be worth around USD 7.3 Billion by 2033, from USD 2.3 Billion in 2023, growing at a CAGR of 12.3% during the forecast period from 2024 to 2033.
Cimzia, scientifically known as certolizumab pegol, is a biologic medication designed for the management of several chronic conditions, including rheumatoid arthritis, chronic disease, and plaque psoriasis. As a tumor necrosis factor (TNF) alpha inhibitor, Cimzia plays a crucial role in modulating the body’s inflammatory response, making it an invaluable asset in the therapeutic arsenal against these debilitating diseases.
Cimzia, a biologic treatment developed by UCB, plays a critical role across multiple end-use industries due to its effectiveness in managing several chronic and inflammatory diseases. According to the American College of Rheumatology, rheumatoid arthritis affects over 1.5 million Americans, highlighting the importance of treatments like Cimzia in rheumatology.
In gastroenterology, Cimzia’s significance is underscored by its use in treating Crohn’s disease, a condition that the Crohn’s and Colitis Foundation of America reports affects approximately 3.1 million Americans. Furthermore, the drug’s approval for moderate to severe plaque psoriasis has expanded its utility into dermatology, serving a significant portion of the 8 million Americans estimated by the National Psoriasis Foundation to have psoriasis.
The financial performance of Cimzia reflects its strong market presence, with global sales surpassing $6 billion in 2020, as reported by IQVIA. This success is part of the broader TNF-alpha inhibitor market, which is projected to reach $40.7 billion, indicating a continued interest and growth in this sector.
Regulatory oversight by the FDA and equivalent international bodies ensures that Cimzia meets stringent safety and efficacy standards. Government initiatives aimed at improving access to high-cost medications like Cimzia can significantly affect its market dynamics. Additionally, international trade regulations impact its distribution and availability across different markets.
Key Takeaways
- Market Size: Cimzia market to reach USD 7.3 Billion by 2033, growing at 12.3% CAGR from 2024 to 2033.
- Therapeutic Significance: Cimzia vital in managing rheumatoid arthritis, Crohn’s disease, and psoriatic arthritis, acting as TNF-alpha inhibitor.
- Market Performance: Global Cimzia sales surpassed $6 billion in 2020, contributing to TNF-alpha inhibitor market’s growth.
- Indication Dominance: Rheumatoid arthritis segment led in 2023, capturing 42.3% market share.
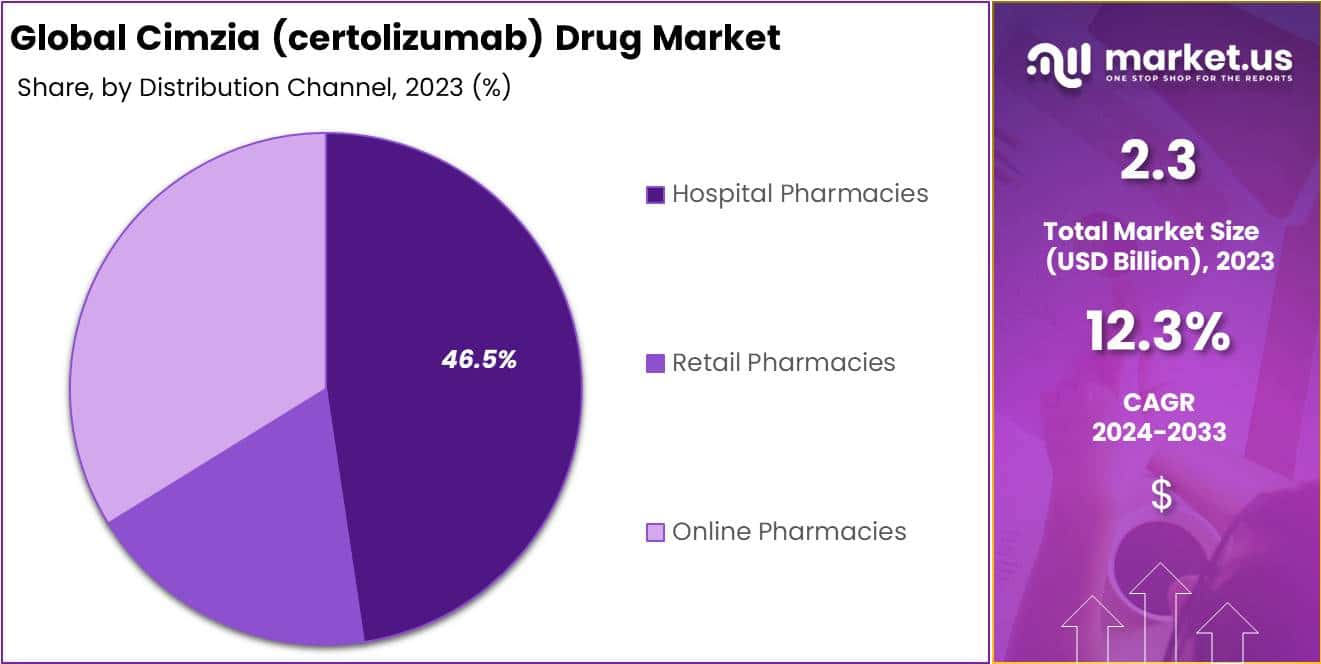
- Distribution Channels: Hospital pharmacies dominate with 46.5% market share, followed by retail and online pharmacies.
- Market Drivers: Rising autoimmune disease prevalence and environmental factors drive Cimzia demand.
- Market Restraints: High costs and accessibility issues pose significant challenges for market growth.
- Market Opportunities: Expansion into emerging markets offers growth potential for Cimzia.
- Trends: Advancements in biologic drug delivery systems aim to enhance patient compliance and treatment outcomes.
- Regional Analysis: North America leads with 60.5% market share, followed by Europe and Asia-Pacific.
Indication Analysis
In 2023, the market for Cimzia (certolizumab) was predominantly led by its application in treating Rheumatoid Arthritis (RA), capturing over 42.3% of the market share. This dominance is primarily due to the global increase in RA cases and Cimzia’s effectiveness in managing this condition by inhibiting tumor necrosis factor-alpha (TNF-α), crucial for reducing inflammation and preventing disease progression.
Additionally, the Psoriatic Arthritis (PsA) segment followed closely, fueled by improved diagnosis rates and the growing preference for biologics in treatment protocols. Cimzia’s ability to enhance physical functions and minimize joint damage in PsA patients has significantly contributed to its market presence.
The drug’s market extends to treating other indications, such as Crohn’s disease and ankylosing spondylitis, showcasing steady growth supported by continuous research and development efforts. These endeavors aim to broaden Cimzia’s therapeutic uses, backed by its favorable safety profile. The market’s future trajectory looks promising, driven by an increasing incidence of autoimmune diseases, advancements in diagnostics, and a shift towards targeted biologic therapies.
Pharmaceutical strategies, including patient support initiatives and research investments, are expected to boost Cimzia’s accessibility and efficacy, solidifying its position in the market and enhancing patient care in autoimmune disease management.
Distribution Channel Analysis
In 2023, the Hospital Pharmacies segment holds dominating position in the Cimzia (certolizumab) Drug Market’s Distribution Channel Segment, securing over 46.5% of the market share. This dominance is largely due to hospital pharmacies’ crucial role in managing the intricate requirements of biologic drugs, including specialized storage and handling.
The need for such drugs to be dispensed under strict medical supervision, as per regulatory guidelines, further cements the importance of hospital pharmacies in this ecosystem. Additionally, Retail Pharmacies carved out a substantial market share, driven by their accessibility and the convenience they offer for long-term treatment regimens, along with their growing capability to manage biologic drugs.
On the other hand, the Online Pharmacies segment, though smaller in comparison, showed the most robust growth in 2023. This acceleration is attributed to the digital shift in healthcare, where patients prefer the convenience of online orders and home delivery for their medications.
Online pharmacies have responded by enhancing their services to include online prescriptions and efficient delivery systems, catering especially to those managing chronic conditions. This segment’s expansion is further facilitated by technological improvements in logistics, ensuring that even temperature-sensitive medications like Cimzia are delivered safely and efficiently. This distribution landscape reflects a patient-oriented approach, evolving with technological advances and regulatory changes to better serve diverse patient needs.
Key Market Segments
Indication
- Psoriatic Arthritis (PsA)
- Rheumatoid Arthritis (RA)
- Other Indications
Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
Drivers
Rising Prevalence of Autoimmune Diseases
The growth of the Global Cimzia (certolizumab) Drug Market can be primarily attributed to the increasing prevalence of autoimmune diseases such as rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease. Certolizumab pegol, marketed as Cimzia, is utilized in the management of these conditions. With the global rise in autoimmune diseases, the demand for effective treatment options like Cimzia is expected to surge, driving the market forward.
- Rising Autoimmune Markers: Research on over 14,000 individuals revealed a significant uptick in antinuclear antibodies (ANA), especially notable in adolescents, whose ANA rates nearly tripled.
- Prevalence of Common Autoimmune Diseases: Over 80 conditions are identified as autoimmune, with type 1 diabetes, rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease among the most prevalent.
- Environmental Factors as Major Contributors: Up to 70% of autoimmune diseases may be linked to environmental factors, including pollutants and dietary components, highlighting the influence of lifestyle and environmental conditions.
- Concern Over Microplastics: Studies suggest adults may ingest over 322,000 microplastic particles annually, a factor potentially contributing to the rise in autoimmune diseases through oxidative stress and inflammation.
Restraints
High Cost and Accessibility Issues
The high cost and accessibility issues serve as significant restraints for the Cimzia (certolizumab pegol) drug market. The economic barriers associated with Cimzia can be attributed to its classification as a biologic drug, which inherently involves complex manufacturing processes leading to higher production and development costs. These costs are frequently passed on to patients, resulting in substantial financial burdens for those requiring this medication.
- It is observed that under most private or employer insurance plans, approximately 69% of Cimzia prescriptions fall into the cost range of $0 to $200 monthly for patients, influenced by the specifics of their insurance coverage and deductible status.
- A considerable segment of Cimzia prescriptions, although less frequent, incurs a monthly cost for patients ranging between $200 and $1,650, highlighting the variability based on individual insurance plan structures.
- For individuals covered by Medicare, it is noted that around 87% of Cimzia prescriptions are priced between $0 to $200 monthly, with the remaining prescriptions averaging a cost of about $1,230 per month.
- Medicaid recipients experience substantially lower copays for Cimzia, with amounts typically ranging from $4.95 to $9.85 monthly, reflecting Medicaid’s structure aimed at reducing patient financial burden.
- The retail price for a single subcutaneous kit of Cimzia (200 mg) without insurance coverage reaches approximately $6,032, posing a significant financial challenge for cash-paying patients, despite the availability of financial assistance programs.
Opportunities
Expansion into Emerging Markets
Emerging markets represent a significant opportunity for the expansion of the Cimzia drug market. With growing healthcare infrastructure and increasing awareness of autoimmune diseases in these regions, there is a rising demand for advanced therapeutics. By focusing on market penetration strategies, including partnerships with local healthcare providers and tailored pricing models, manufacturers have the opportunity to tap into new consumer bases and drive market growth.
Trends
Advancements in Biologic Drug Delivery
The trend of advancements in biologic drug delivery systems is significantly shaping the global market for Cimzia (certolizumab pegol), with a notable focus on enhancing patient compliance and treatment outcomes. These innovations include prefilled syringes, auto-injectors, and wearable injectors, which offer improved efficacy, reduced side effects, and increased convenience for patients.
- Large Volume Wearable Injectors (LVWIs) are revolutionizing drug administration by enabling self-injection of viscous drugs at home or work, aiming for patient convenience and reduced healthcare costs. Predicted to grow at a 23% CAGR until 2024, LVWIs mark a significant shift toward integrating delivery systems early in drug development to enhance patient outcomes and differentiate products in the market.
- Advancements in subcutaneous delivery technologies, notably the use of rHuPH20 for improved permeation, are making it possible to administer higher doses more effectively and comfortably. This evolution in drug delivery technologies is broadening the spectrum of manageable volumes and viscosities, leading to shorter administration times and enhanced patient experiences.
- The biologics market, significantly influenced by in-house production, which made up 84.87% of the market in 2022, benefits from direct control over the complex manufacturing processes of biologic drugs like Cimzia. This method ensures strict quality oversight.
- The outsourcing sector is witnessing growth, driven by the expertise and advanced capabilities of Contract Development and Manufacturing Organizations (CDMOs). This trend reflects the industry’s pursuit of innovation and increased production capacities to meet escalating market demands.
Regional Analysis
In 2023, the Cimzia (certolizumab) Drug Market in North America demonstrated a commanding presence, securing a market share exceeding 60.5% with a valuation of USD 1.39 billion. This dominance is attributed to an advanced healthcare infrastructure, widespread patient education programs, and favorable reimbursement policies.
The region’s market is further strengthened by the active R&D endeavors undertaken by leading pharmaceutical entities. Following North America, Europe maintained its position as the second-largest market. This is primarily due to the rising prevalence of autoimmune disorders like rheumatoid arthritis and Crohn’s disease, alongside robust healthcare systems and regulatory support ensuring access to cutting-edge treatments.
On the other hand, the Asia-Pacific region is on track to exhibit the most rapid growth in the coming years, fueled by enhancements in healthcare facilities, increased healthcare spending, and heightened awareness of autoimmune diseases. The introduction of generic drug versions is also expected to boost market growth in this area.
Meanwhile, Latin America and the Middle East & Africa are predicted to see modest growth, driven by improvements in healthcare services, government initiatives to improve healthcare reach, and partnerships between local and international firms to distribute advanced treatments. The global outlook for Cimzia remains positive, with ongoing R&D and strategic collaborations poised to unlock new market opportunities and improve drug accessibility worldwide.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
In the analysis of the Cimzia (certolizumab) drug market, understanding the competitive landscape and strategic positioning is vital. Cimzia, or certolizumab, is a biologic medication used to treat chronic inflammatory conditions like rheumatoid arthritis and Crohn’s disease by targeting tumor necrosis factor alpha (TNFα). UCB Pharma, the original developer and patent holder, dominates the market with its extensive experience and strategic initiatives.
However, competition exists from other TNF inhibitors like Humira (adalimumab) and Enbrel (etanercept), supported by AbbVie and Amgen, respectively. Biosimilars, offering similar benefits at lower costs, pose a growing threat. To counter, UCB Pharma and others invest in innovation, exploring new formulations and therapeutic indications. Building strong relationships with healthcare providers and patient advocacy groups is key to enhancing brand loyalty and market penetration. Despite challenges, the market remains dynamic, with UCB Pharma adapting to maintain its position amidst evolving competition and healthcare dynamics.
*Note: Final report will include key players in the market.
Report Scope
Report Features Description Market Value (2023) USD 2.3 Bn Forecast Revenue (2033) USD 7.3 Bn CAGR (2024-2033) 12.3% Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, COVID-19 Impact, Competitive Landscape, Recent Developments Segments Covered By Indication (Psoriatic Arthritis (PsA), Rheumatoid Arthritis (RA), Other Indications), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), Regional Analysis North America – The US, Canada, & Mexico; Western Europe – Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, & Rest of Western Europe; Eastern Europe – Russia, Poland, The Czech Republic, Greece, & Rest of Eastern Europe; APAC – China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, & Rest of APAC; Latin America – Brazil, Colombia, Chile, Argentina, Costa Rica, & Rest of Latin America; Middle East & Africa – Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, & Rest of MEA Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is the size of the Cimzia (certolizumab) Drug market in 2023?The Cimzia (Certolizumab) Drug market size is USD 2.3 billion in 2023.
What is the projected CAGR at which the Cimzia (certolizumab) Drug market is expected to grow at?The Cimzia (certolizumab) Drug market is expected to grow at a CAGR of 12.3% (2024-2033).
List the segments encompassed in this report on the Cimzia (certolizumab) Drug market?Market.US has segmented the Cimzia (certolizumab) Drug market by geographic (North America, Europe, APAC, South America, and Middle East and Africa). By Indication the market has been segmented into Psoriatic Arthritis (PsA), Rheumatoid Arthritis (RA), Other Indications. By Distribution Channel the market has been segmented into Hospital Pharmacies, Retail Pharmacies, Online Pharmacies.
Which region is more appealing for vendors employed in the Cimzia (certolizumab) Drug market?North America is expected to account for the highest revenue share of 60.5% and boasting an impressive market value of USD 1.39 billion. Therefore, the Cimzia (certolizumab) Drug industry in North America is expected to garner significant business opportunities over the forecast period.
Name the key areas of business for Cimzia (certolizumab) Drug?The US, Canada, India, China, UK, Japan, & Germany are key areas of operation for the Cimzia (certolizumab) Drug Market.
 Cimzia (certolizumab) Drug MarketPublished date: March 2024add_shopping_cartBuy Now get_appDownload Sample
Cimzia (certolizumab) Drug MarketPublished date: March 2024add_shopping_cartBuy Now get_appDownload Sample -
-










