Quick Navigation
Introduction: Importance of Pharmacovigilance
The pharmacovigilance market is gaining importance as a key element in global healthcare systems. It ensures drug safety through the detection, evaluation, and prevention of adverse drug reactions (ADRs). The growing burden of chronic diseases has increased long-term medicine use, raising the need for stronger monitoring. As pharmaceutical consumption rises globally, healthcare providers and regulators are prioritizing safety. This shift is driving the expansion of pharmacovigilance systems and creating opportunities for new solutions to improve patient outcomes.
The growth of the pharmacovigilance market is supported by strict regulatory frameworks and rising awareness of patient safety. Authorities worldwide have mandated reporting and monitoring standards, which pharmaceutical companies must follow. At the same time, technological advances such as artificial intelligence and big data analytics are transforming pharmacovigilance practices. These tools improve adverse event detection and streamline compliance processes. As a result, the market is positioned for significant growth, supported by regulatory demands, technology adoption, and global healthcare priorities.
Market Growth & Forecast
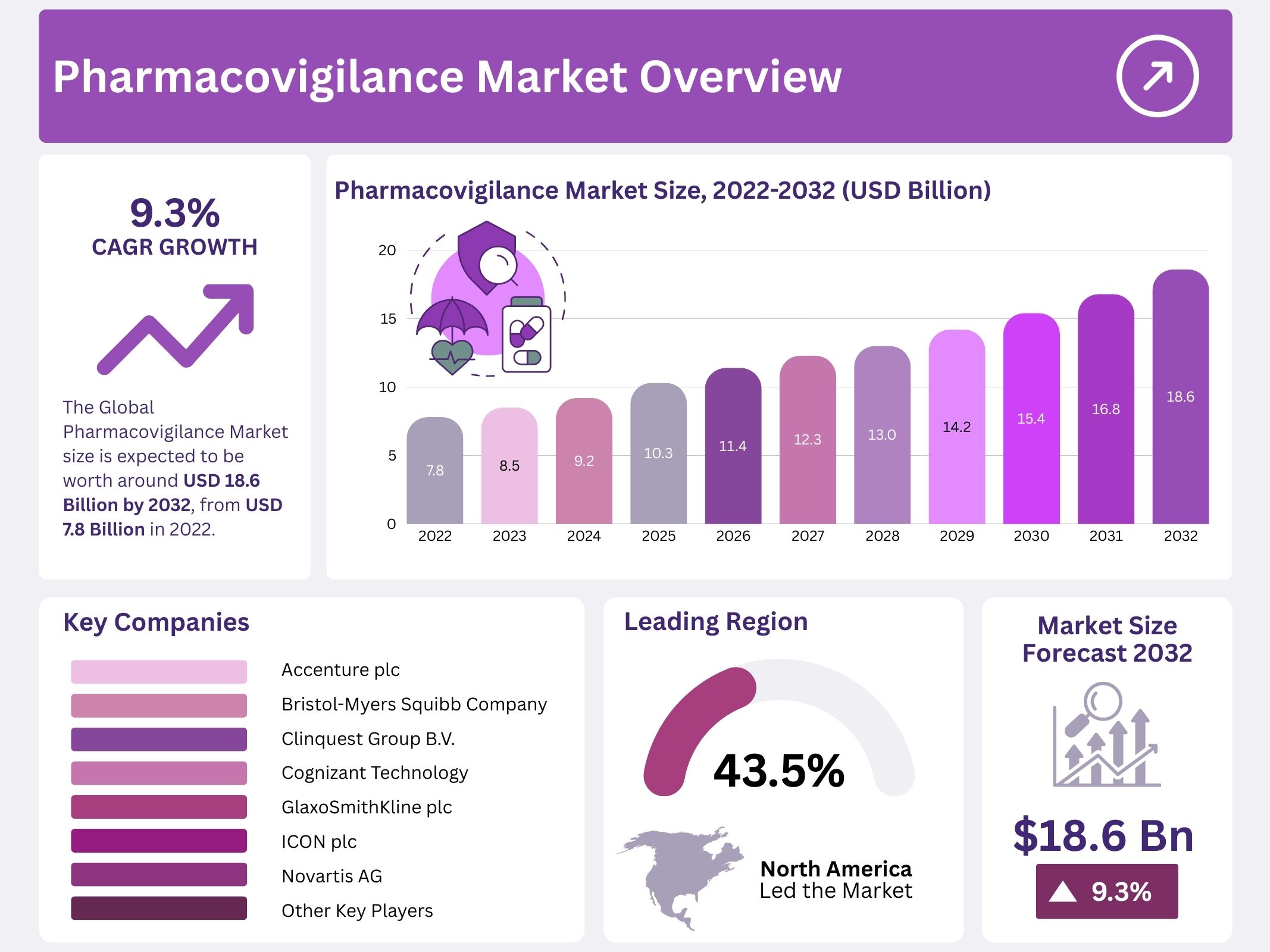
The global pharmacovigilance market was valued at USD 7.8 billion in 2022. It is projected to grow at a compound annual growth rate (CAGR) of 9.3% from 2023 to 2032. By 2032, the market is expected to reach USD 18.6 billion.
The growth in drug development pipelines has been supported by increasing innovation and investment across therapeutic areas. This has created a higher demand for robust monitoring systems that ensure patient safety. The introduction of strict safety regulations has further reinforced the need for pharmacovigilance solutions. Regulatory authorities require companies to adopt strong compliance frameworks. As a result, organizations have been investing in advanced monitoring tools. These tools are designed to detect risks early, reduce liabilities, and strengthen trust in drug safety practices.
Post-marketing surveillance plays a critical role in ensuring drug safety after approval. Most unexpected adverse drug reactions (ADRs) are reported during Phase IV clinical trials. This highlights the importance of continuous safety monitoring. Comprehensive pharmacovigilance systems provide real-time data collection and reporting capabilities. These systems also allow timely corrective actions to be implemented. Growing awareness among stakeholders has further increased adoption. The focus remains on patient safety, regulatory compliance, and risk management. This has positioned pharmacovigilance as a key growth driver in the healthcare sector.
Key Takeaways
- The global pharmacovigilance market is projected to reach USD 19 billion by 2032, advancing steadily with a compound annual growth rate of 9.3%.
- The rising prevalence of chronic diseases significantly drives drug consumption worldwide, thereby increasing demand for pharmacovigilance services to ensure patient safety and effective monitoring.
- Spontaneous reporting holds the largest share in pharmacovigilance, supported by its advantages in drug comparison and data simulation for safety evaluations.
- Cohort event monitoring is gaining popularity, driven by advanced data mining techniques and its ability to track medicine safety across both old and new drugs.
- Targeted spontaneous reporting is expected to grow fastest, primarily due to government-led initiatives encouraging diverse, structured, and innovative reporting methodologies in pharmacovigilance practices.
- Electronic health records are increasingly used in pharmacovigilance, particularly for identifying potential risks following hospital discharge, thus fueling growth in this segment.
- Phase IV post-marketing surveillance dominates pharmacovigilance activities, being crucial in identifying and monitoring unexpected adverse drug reactions that emerge after commercial drug approval.
- Contract outsourcing represents the fastest-growing service model, as pharmaceutical companies benefit from lower operational costs, enhanced flexibility, and resource-sharing opportunities offered by external providers.
- Oncology remains a key growth driver for pharmacovigilance, as monitoring cancer drug safety is essential due to the high incidence of severe side effects.
- Pharmaceutical companies continue to hold a substantial market share, supported by their rising adoption of pharmacovigilance services to meet regulatory requirements and ensure drug safety.
Key Trends Shaping the Market
Integration of AI and Digital Technologies
The adoption of artificial intelligence (AI), machine learning, and big data analytics is transforming pharmacovigilance. Automated signal detection, real-time monitoring, and predictive risk analysis are enhancing the efficiency of ADR reporting and case management.
Outsourcing Models Gain Momentum
Contract outsourcing is becoming the fastest-growing service provider segment. Lower operational costs, scalability, and access to specialized expertise are driving pharmaceutical and biotechnology companies to outsource pharmacovigilance activities.
Strengthening Regulations and Compliance
Global regulatory authorities are enforcing stricter guidelines to ensure drug safety. Agencies such as the U.S. FDA and the European Medicines Agency (EMA) are mandating continuous safety assessments, which is propelling the demand for advanced pharmacovigilance systems.
Expanding Clinical Trial Complexity
As clinical trials become more global and diverse, pharmacovigilance systems are increasingly necessary to manage large-scale safety data. This trend is particularly evident in oncology and rare disease therapies, where treatment-related risks require close monitoring.
Challenges & Opportunities
Challenges
- High Costs of Compliance: Maintaining compliance with international regulatory standards requires significant financial investment.
- Data Management Complexity: Handling diverse safety data from multiple geographies and sources creates operational challenges.
- Shortage of Skilled Professionals: A global talent gap in pharmacovigilance expertise is impacting service quality.
Opportunities
- Rising Chronic Disease Burden: Increasing cases of cancer, cardiovascular disorders, and neurological diseases drive higher drug consumption, expanding the need for safety monitoring.
- Electronic Health Records (EHR) Utilization: Growing adoption of EHRs enables efficient risk identification post-hospital discharge.
- Emerging Markets Growth: Asia-Pacific and Latin America present untapped opportunities, supported by government initiatives to strengthen pharmacovigilance infrastructure.
Market Segmentation
The pharmacovigilance market is dominated by spontaneous reporting, which holds the largest revenue share due to its wide application in drug safety monitoring. It plays a major role in detecting new, rare, and serious adverse drug reactions (ADRs) and is also considered cost-effective. Cohort event monitoring (CEM) ranks as the second-largest type, supported by the integration of statistical tools and data mining systems, particularly electronic health records, enhancing detection of clinical events.
Within the product life cycle, Phase IV (post-marketing) maintains a leading share. This dominance is attributed to extensive post-marketing surveillance and the rising incidence of ADRs. The stage is critical as it allows unanticipated drug reactions to be identified after commercialization, making data collected during this phase highly valuable. Meanwhile, Phase III is projected to grow at 10.5%, driven by its role in drug efficacy validation and safety evaluation before market entry.
Spontaneous reporting systems (SRSs) are the most widely used process flow, as healthcare professionals voluntarily notify regulators of suspected ADR cases. However, advancements in big data and artificial intelligence are reshaping signal detection and evaluation. Case data management is anticipated to grow at the fastest pace, fueled by adverse event information generated through clinical trials, literature, and post-marketing programs, thereby supporting more efficient pharmacovigilance operations globally.
By therapeutic area, oncology holds the highest growth potential due to the side effects associated with cancer drugs. Monitoring safety is crucial as both targeted therapies and systemic treatments carry significant toxicity risks. Rising cancer incidence is driving R&D and pharmacovigilance service demand. Pharmaceutical companies remain the largest end-users, supported by regulatory mandates and recalls, such as Aurobindo Pharma’s product withdrawal in 2022. Strategic partnerships, such as IQVIA’s collaboration with NRx Pharmaceuticals, further strengthen this segment.
Regional Outlook
North America dominates the pharmacovigilance market due to its strong pharmaceutical and medical device industry. The region’s leadership is supported by robust regulatory frameworks, advanced healthcare infrastructure, and high drug consumption. Rising drug abuse and related adverse reactions have increased the demand for monitoring systems. These factors, combined with the presence of key players, have contributed to North America holding the largest revenue share in the global market.
The market expansion in North America is driven by increasing investment in drug development and innovation. A significant rise in clinical trials has been observed as companies focus on novel therapies. This has created a growing need for post-marketing surveillance and risk management. The demand for pharmacovigilance services is also fueled by the high production of drugs, making the region a key growth contributor within the global industry.
Europe follows closely, supported by EMA regulations and a strong pharmaceutical base. Meanwhile, Asia-Pacific is projected to grow at the fastest rate, with rising healthcare spending and clinical research activities. Government initiatives to improve patient safety also support this growth. Latin America and the Middle East present emerging opportunities, where evolving regulations and higher patient awareness are expected to drive adoption. These regions collectively enhance the overall global market outlook.
Competitive Landscape
The global pharmacovigilance market is fragmented, with key players focusing on strategic collaborations, technological integration, and outsourcing partnerships. Major players include:
Market Key Players
- Accenture plc
- Bristol-Myers Squibb Company
- Clinquest Group B.V.
- Cognizant Technology Solutions Corporation
- GlaxoSmithKline plc
- ICON plc
- Novartis AG
- Hoffmann-La Roche Ltd.
- PAREXEL International Corporation
- Pfizer Inc.
- ICON plc
- Wipro Limited
These companies are leveraging digital tools, expanding global operations, and investing in research to strengthen their pharmacovigilance capabilities.
Analyst Quote
“Pharmacovigilance is no longer limited to regulatory compliance. It has become a strategic priority for pharmaceutical companies. The integration of AI, increased outsourcing, and expanding drug pipelines will continue to shape the market’s future,” said a senior market analyst at Market.us.
Future Outlook
The pharmacovigilance market is poised for sustained growth over the next decade. Post-marketing surveillance will remain central, supported by advanced digital platforms and global harmonization of drug safety standards. Oncology will continue to dominate therapeutic monitoring, while the rapid adoption of targeted spontaneous reporting and EHR-based monitoring will accelerate efficiency. Companies that embrace outsourcing models and digital transformation are expected to achieve long-term competitiveness in this evolving market.
Report Availability
The full report on the Global Pharmacovigilance Market provides comprehensive insights into growth drivers, challenges, opportunities, and competitive dynamics. It includes detailed segmentation analysis and regional forecasts.
About Us
Market.us, powered by Prudour Pvt. Ltd., is a leading market research and consulting company. The firm provides in-depth industry reports, syndicated research, and customized consulting services. Its expertise spans multiple sectors, including healthcare, technology, and consumer goods. With a global presence and a team of skilled analysts, Market.us delivers actionable intelligence to support strategic business decisions.
Media Contact
Contact No: +1 718 874 1545 (International), +91 78878 22626 (Asia)
Email: [email protected]










