Quick Navigation
Overview
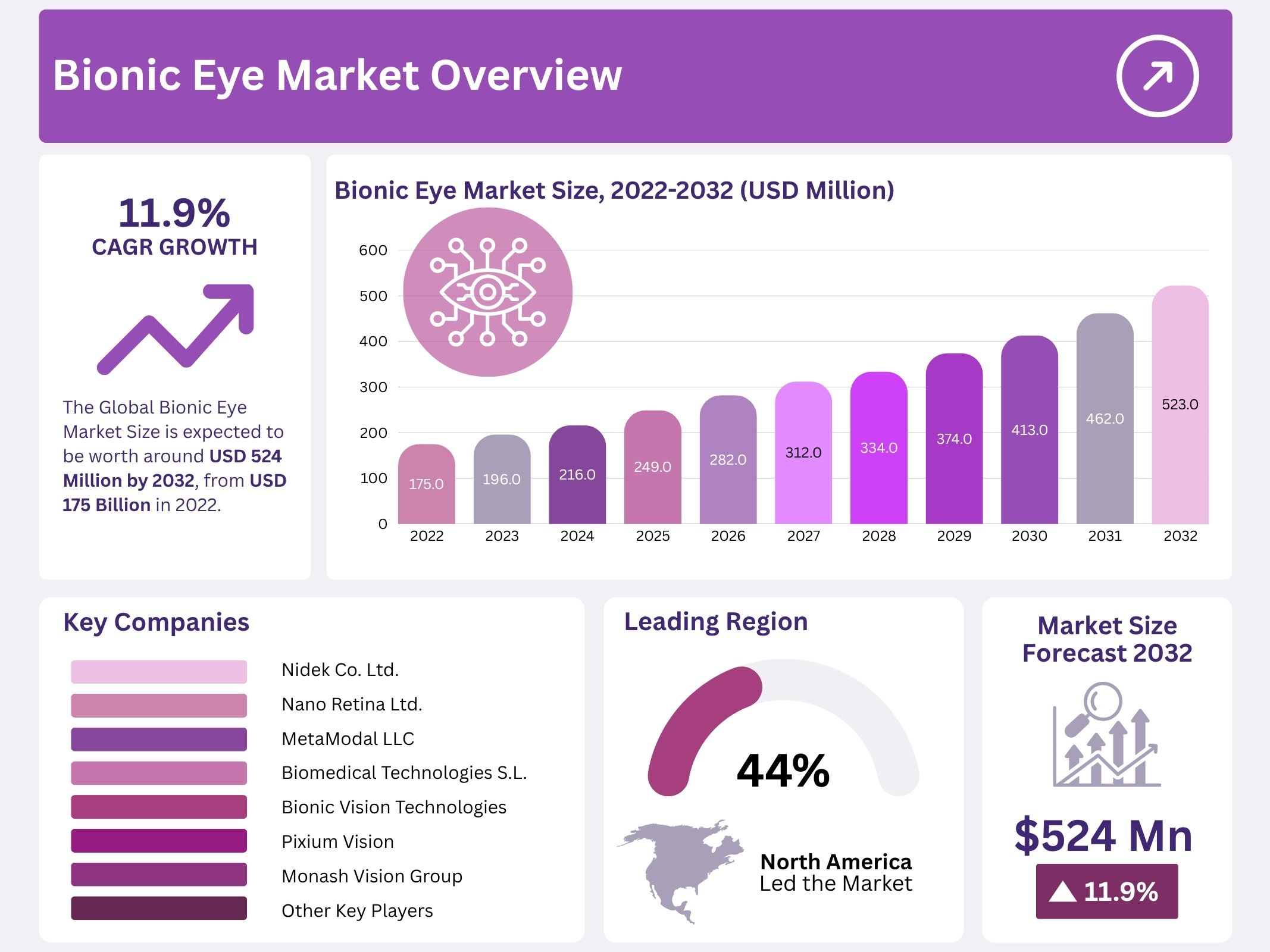
The Global Bionic Eye Market is expanding due to increasing demand for advanced visual restoration solutions. The market value is projected to reach USD 524 million by 2032. It was estimated at USD 175 million in 2022. A CAGR of 11.9% is expected during 2024 to 2033. Growth is supported by a rising burden of vision loss and continuous improvements in neuro-engineering. Age-related degenerative conditions are rising among the elderly. This trend creates a strong need for ocular prosthetics. Healthcare systems across developed countries are focusing on new treatments that restore visual function. As a result, technology adoption and commercialization are moving forward.
Global visual impairment levels are rising. International health bodies report that more than 250 million people suffer from vision loss. Major causes include age-related macular degeneration, glaucoma, and diabetic retinopathy. Aging populations in the United States, Germany, and Japan are more vulnerable to these diseases. This demographic shift has increased the need for innovative treatment options. Bionic eye solutions are being positioned as an alternative when conventional therapies fail. Improved clinical awareness has helped patients explore advanced medical devices. As vision-related disabilities increase, demand for bionic implants is expected to grow steadily.
Advances in retinal implants, microelectronics, and neural interface systems have improved device performance. Artificial intelligence enables better image interpretation and enhanced visual outcomes. The introduction of biocompatible materials reduces surgical risks and improves durability. These technological upgrades are boosting confidence among healthcare providers. New entrants and established manufacturers are investing in research to expand product pipelines. Continuous improvements support broader clinical applications. Better visual acuity results contribute to increased patient acceptance. As innovation accelerates, cost efficiency and reliability are expected to improve, driving stronger market penetration.
Government support plays an important role in the commercialization of bionic eye devices. Regulatory approvals in North America and Europe have increased market access. Select healthcare systems have introduced reimbursement frameworks to make the technology affordable. Rising healthcare expenditure is reinforcing development activities. Public institutions and venture capital groups are funding sensory restoration projects. These investments support both early-stage innovations and large-scale production. Strategic programs encourage clinical trials and product launches. Better coverage policies will help increase patient uptake. The combination of strong funding and supportive regulations ensures long-term industry growth.
Key Takeaways
- The global bionic eye market is expected to reach USD 524 million by 2032, reflecting strong expansion from 2022 levels with an 11.9% CAGR.
- External bionic eye solutions dominate with 57.4% market share, indicating higher demand and improved vision restoration outcomes supported by continuous product innovation and technology upgrades.
- Electronic eye technology maintains a leading 67% share, driven by successful clinical trials, increasing adoption rates, and sustained performance improvements in visually impaired patient populations worldwide.
- Hospitals represent 62.2% of the market due to skilled ophthalmologists, advanced infrastructure, and strong collaboration with device manufacturers for deployment of bionic implants.
- Rising global incidence of severe vision loss and eye-related disorders plays a major role in accelerating demand for advanced bionic eye restoration solutions worldwide.
- High treatment costs significantly limit adoption, with accessibility remaining largely restricted to developed nations where reimbursement and technology availability levels are higher.
- Growing research investments and supportive government initiatives foster faster technological progress, opening new commercial opportunities for innovative bionic eye devices in future markets.
- North America leads with 44% share due to superior healthcare resources, while Asia Pacific grows rapidly following new product launches and rising patient adoption.
Regional Analysis
North America dominates the bionic eye market. The region holds a market revenue share of about 44% during the forecast period. The strong position is supported by advanced healthcare systems. Robust reimbursement structures are also present. Leading medical research organizations are located in the region. Continuous technological advancement is encouraged by well-developed infrastructure. Supportive regulatory systems help in faster product approvals. Major companies invest heavily in innovation. This contributes to commercial adoption. High awareness among patients leads to early acceptance of bionic eye implants. Growth remains steady due to strong demand.
Access to specialized healthcare services supports industry expansion. Hospitals and clinics are well equipped. Skilled professionals manage complex surgical procedures. Research activities are intensive across the United States and Canada. Many trials for next-generation visual prosthetics are ongoing. Partnerships between medical institutes and technology providers are rising. Funding from governments and private organizations is high. These efforts increase device performance and safety. As a result, the acceptance of bionic eyes improves. Strong patient support initiatives and diagnostics strengthen market penetration further. Innovation continues to drive market growth.
Printing electronics on curved surfaces was a major challenge. New technology now addresses that issue effectively. Solar-powered retinal implants are under development. These products aim to treat vision loss. The target patient group includes individuals with degenerative retinal disorders. Retinitis pigmentosa is a major focus area. The technology attempts to restore partial vision. Improved biocompatibility is being achieved. Device miniaturization supports comfort. Production capabilities are expanding gradually. More availability of efficient components is expected. Demand for vision restoration solutions rises. The market outlook remains positive.
Asia Pacific is emerging as the second fastest growing region. The market expansion is driven by increasing product launches. Many novel bionic eye implants enter the market. Healthcare access is improving in major countries. Governments support medical technology advancements. Awareness of treatment options is increasing. The patient pool suffering from retinal disorders is large. Investment in research is rising. Medical tourism also drives demand. Pricing strategies help increase adoption. Local players expand production capabilities. Technological upgrades encourage regional competitiveness. Therefore, Asia Pacific shows high future growth potential.
Segmentation Analysis
The bionic eye market is segmented into external and implanted eyes. The external eye segment holds a leading share of about 57.4%. Growth has been driven by new technologies that improve patient outcomes. An example includes the Orion bionic eye with upgraded external hardware. It offers face detection and 3D vision through stereo cameras attached to eyewear. The latest model supports obstacle detection and navigation features. Increasing cases of age-related macular degeneration, which causes blurred vision, are expected to boost the adoption of bionic eyes.
The market is categorized into electronic and mechanical eye technologies. The electronic segment holds around 67% share. This growth can be attributed to rising clinical trials and strong adoption of electronic implants. These systems apply neural stimulation in the eye. A video camera captures visual information. The signals are decoded by a wearable battery-powered device. They are then converted into electrical stimulation and sent to the implanted device. Mechanical implants are growing quickly. They treat severe macular degeneration and enlarge visual projections on the retina.
The market for bionic eyes is divided into hospitals, ophthalmic clinics, and other end-users. Hospitals account for nearly 62.2% share. Hospitals are the primary care centers for vision loss and provide skilled ophthalmologists and advanced equipment. Higher medical coverage encourages more surgery procedures. Positive surgical outcomes support acceptance of the technology. Collaborations between hospitals and product developers to conduct clinical trials also contribute to the segment’s expansion. These factors are increasing the demand for bionic eye implants in hospital settings worldwide.
Key Market Segments
By Eye Type
- External Eye
- Implanted Eye
By Eye Technology
- Electronic
- Mechanical
By End-User
- Hospitals
- Ophthalmic Clinics
- Other End-Users
Key Players Analysis
The competitive landscape of the bionic eye market is characterized by steady innovation and global business expansion. The development of advanced visual prosthetic technologies is being pursued to meet unmet clinical needs. Companies are implementing strategic policies to strengthen their presence in international markets. Investments in research infrastructure and collaborations with healthcare institutions are increasing. The focus is on improving device performance and patient outcomes. Product commercialization remains a priority. Second Sight Medical Products LLC and Nano Retina Ltd. are strengthening their product pipelines to maintain competitiveness and support sustainable growth.
Commercial expansion strategies are being adopted to increase market reach. Key players are actively pursuing mergers and acquisitions to broaden their product portfolios. This approach enhances access to innovative implant systems and surgical solutions. Nidek Co. Ltd. and Pixium Vision continue to advance new-generation retinal prostheses with enhanced capabilities. These companies aim to accelerate regulatory approvals and global distribution. Improved technology performance supports greater patient adoption. Industry participants are also targeting markets with larger unmet demand. This supports higher revenue potential and broader patient coverage.
Marketing strategies in the bionic eye market are increasingly focused on awareness creation and product visibility. Companies highlight technological strengths and clinical benefits to improve interest among ophthalmologists and patients. Partnerships with hospitals enable demonstration of device effectiveness. Bionic Vision Technologies and MetaModal LLC are enhancing strategic collaborations to increase brand value and clinical acceptance. Communication efforts emphasize safety, reliability, and restoration of partial vision. Expansion into emerging economies is accelerating. High investment in clinical trials supports product credibility and strengthens market position.
The market remains fragmented due to the presence of several regional and local manufacturers. Competitive pressure is rising as established players leverage strong distribution capabilities. New entrants are encouraged by ongoing research advancements. Biomedical Technologies S.L., Monash Vision Group, and other innovators are focusing on cost-effective devices and scalable manufacturing. Companies are diversifying product offerings to reduce dependency on single technologies. Strategic alignment with regulatory guidelines is vital. Strong commitment to innovation is expected to support long-term growth and contribute to broader market development.
Market Key Players
- Second Sight Medical Products LLC
- Nidek Co. Ltd.
- Nano Retina Ltd.
- MetaModal LLC
- Biomedical Technologies S.L.
- Bionic Vision Technologies
- Pixium Vision
- Monash Vision Group
- Other Key Players
Conclusion
The global bionic eye market is showing strong long-term growth, supported by rising cases of severe vision loss and continuing progress in visual restoration technology. Demand is increasing as more patients seek advanced solutions when traditional treatments do not work. The development of smarter implants, improved materials, and better surgical outcomes is helping to build confidence in this field. Supportive policies, research funding, and growing clinical awareness also encourage adoption in major healthcare systems. Although high costs limit access in many regions, ongoing innovation is expected to improve affordability over time. The market is positioned to expand steadily as technology becomes more reliable and widely available.










